Surface Tension Formula, Definition & SI Unit- All you need to know in 2024
- 492
- 0
- 0
Surface Tension Formula
Understanding surface tension and its associated formula holds paramount importance for students across scientific disciplines. Surface tension, defined as the force per unit length acting on the surface of a liquid, is a fundamental property governing the behavior of liquids. It influences phenomena ranging from the formation of droplets and bubbles to capillary action. Knowledge of surface tension and its formula is essential for comprehending these behaviors and their underlying principles. Moreover, surface tension plays a critical role in diverse fields such as fluid mechanics, chemistry, and material science. Students need to grasp the concept and formula to analyze and solve problems in these disciplines effectively.

Introduction
Surface tension & surface tension formula is a fascinating phenomenon that affects our everyday lives, from the way water beads up on a leaf to the formation of droplets on a spider’s web. Understanding surface tension can seem daunting at first, but with the right approach, anyone can grasp its concepts, including its formula.
Surface tension is like a force that makes the surface of liquids act like they’re covered in a thin, stretchy skin. Think about when you fill a glass with water and it’s so full that it’s about to spill, but you can still add a few more drops without it overflowing right away. That’s because of surface tension. It’s also what makes spilled mercury form into little round beads. So, surface tension is this cool thing about liquids where their surface tries to shrink to be as small as possible.
What is Surface Tension?
Surface tension describes what happens when the surface of a liquid meets another substance, like another liquid. The liquid tries to make its surface as small as possible, acting almost like a stretchy sheet. The definition can be summed up as follows: surface tension is caused by the attraction between the particles on the surface of a liquid and the particles inside the liquid. This attraction makes the liquid’s surface want to minimize its area.
Surface tension isn’t just about the liquid itself. It also depends on what the liquid is touching—whether it’s another liquid, a solid, or even a gas. Think of surface tension as the energy needed to remove a layer of molecules from the liquid’s surface. It’s like how much work you’d have to do to pull apart those molecules.
We usually measure surface tension in dynes per centimeter (dynes/cm). This measures the force required to break a film of the liquid that’s 1 centimeter long.
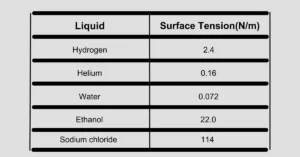
The table provided below displays the surface tension of different liquids:
Cause of Surface Tension
Surface tension arises from intermolecular forces like the Van der Waals force, which attract liquid particles to each other. These forces pull the particles toward the bulk of the liquid along its surface. Surface tension can be described as the relationship between the surface force (F) and the length (L) over which this force operates.
Mathematically, surface tension can be expressed as follows:
T=F/L
In this context of surface tension formula:
( F) represents the force exerted per unit length,
(L) signifies the distance over which this force operates, and
(T) denotes the surface tension of the liquid.
Surface Tension Formula, Definition & SI Unit
What is the Unit of Surface Tension?
The SI unit for surface tension is Newton per meter (N/m). Check other units in the table provided below.
SI Unit: N/m
CGS Unit: dyn/cm

Dimension of Surface Tension
We all know the surface tension is given by the formula of,
Surface tension = F/L
All we know is F = ma, substituting the given value in the given equation, we get
=ma/L
Equating all the fundamental and given quantities into the equation, we can get
=MLT-2L-1
Solving further, we get
=MT-2
In the end, the needed dimensional formula of surface tension is MT-2.
Examples of Surface Tension
Surface tension is like a “skin” on the surface of water that makes it act in certain ways. Here are some cool examples that show how it works:
- Water Striders: These are bugs that can walk on water without sinking because they’re so light and the surface tension of water holds them up.
- Floating Needle: You can carefully place a needle on the surface of water, and it won’t sink because of the surface tension.
- Rainproof Tents: Tents made with special materials use the surface tension of water to keep you dry when it rains. The water’s surface tension fills up tiny holes in the tent material, keeping water out.
- Jaundice Test: Doctors use the surface tension of liquids to help diagnose illnesses like jaundice, a liver problem.
- Cleaning with Soap: Soap makes it easier to clean clothes because it lowers the surface tension of water, helping it spread out and wash away dirt better.
- Cold Water Washing: Washing with cold water uses less energy and still gets clothes clean, thanks to surface tension helping soap do its job.
- Bubbles: Soap bubbles are round because of surface tension. It’s like a force that pulls the bubble into a sphere shape.
Example
Let’s say we have a liquid and a force of 7 Newtons acting over a length of 2 meters.
So, we plug these values into the formula:
Given,
- F = 7 N
- L = 2 m
According to the formula,
T = F/L
⇒ T = 7/2
⇒ T = 3.5 N/m
This means the surface tension of the liquid is 3.5 Newtons per meter.
Methods & Measurements
Now, let’s talk about some methods to measure surface tension:
- Spinning Drop Method: This involves spinning a tiny drop of liquid in another liquid and measuring its shape.
- Pendant Drop Method: Here, a drop of liquid hangs from a tube, and its shape helps determine surface tension.
- Du Noüy–Padday Method: It uses a special instrument to measure the force needed to separate a platinum ring from the liquid’s surface.
- Wilhelmy Plate Method: A plate is immersed in the liquid, and the force needed to pull it out is measured.
- Capillary Rise Method: This method observes how high a liquid rises in a small tube (capillary) to measure surface tension.
- Bubble Pressure Method: It measures the pressure inside a bubble to calculate surface tension.
- Resonant Oscillations Method: It measures the vibrations of a liquid drop to determine surface tension.
- Sessile Drop Method: This method studies the shape of a drop of liquid resting on a solid surface to find surface tension.
These methods help scientists accurately measure surface tension in different liquids.
Conclusion
In summary, surface tension is a property of liquids that arises due to the cohesive forces between molecules at the surface. It is measured in units of force per unit length. The formula commonly used to calculate surface tension is γ = F / L, where γ represents surface tension, F is the force acting perpendicular to the surface, and L is the length over which the force acts. This formula helps quantify the strength of surface tension in various liquids and provides insight into their behaviors and interactions.
FAQs
1. How do you find surface tension?
Surface tension is found using the drop method by measuring the weight or volume of drops from a capillary tube, and then calculating surface tension based on this data.
2. What is surface tension?
Surface tension is the cohesive force between molecules at a liquid’s surface, causing it to minimize surface area. It’s measured in force per unit length.
3. What is surface tension and its SI unit?
Surface tension is the cohesive force between molecules at a liquid’s surface, causing it to minimize surface area. Its SI unit is Newton per meter (N/m).
4. Why do we calculate surface tension?
We calculate surface tension to understand the cohesive forces between molecules at a liquid’s surface, predict behaviors like capillary action, and design processes in various industries.
5. What is the unit of viscosity?
The unit of viscosity is the Pascal-second (Pa·s) in the International System of Units (SI). Another commonly used unit is the centipoise (cP).
Also Read:
Explore the Best Career Options After 12th: A Comprehensive Guide In 2024
Understanding UPSC Exam Eligibility Educational Qualifications in 2024
References:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10132450/
https://ncert.nic.in/textbook.php?leph1=0-8
Disclaimer: Surface Tension Formula, Definition & SI Unit – All You Need to Know in 2024
This article provides general information about surface tension. For detailed understanding and application, consult educational resources or experts. We are not responsible for any misinterpretation or application of the information.
